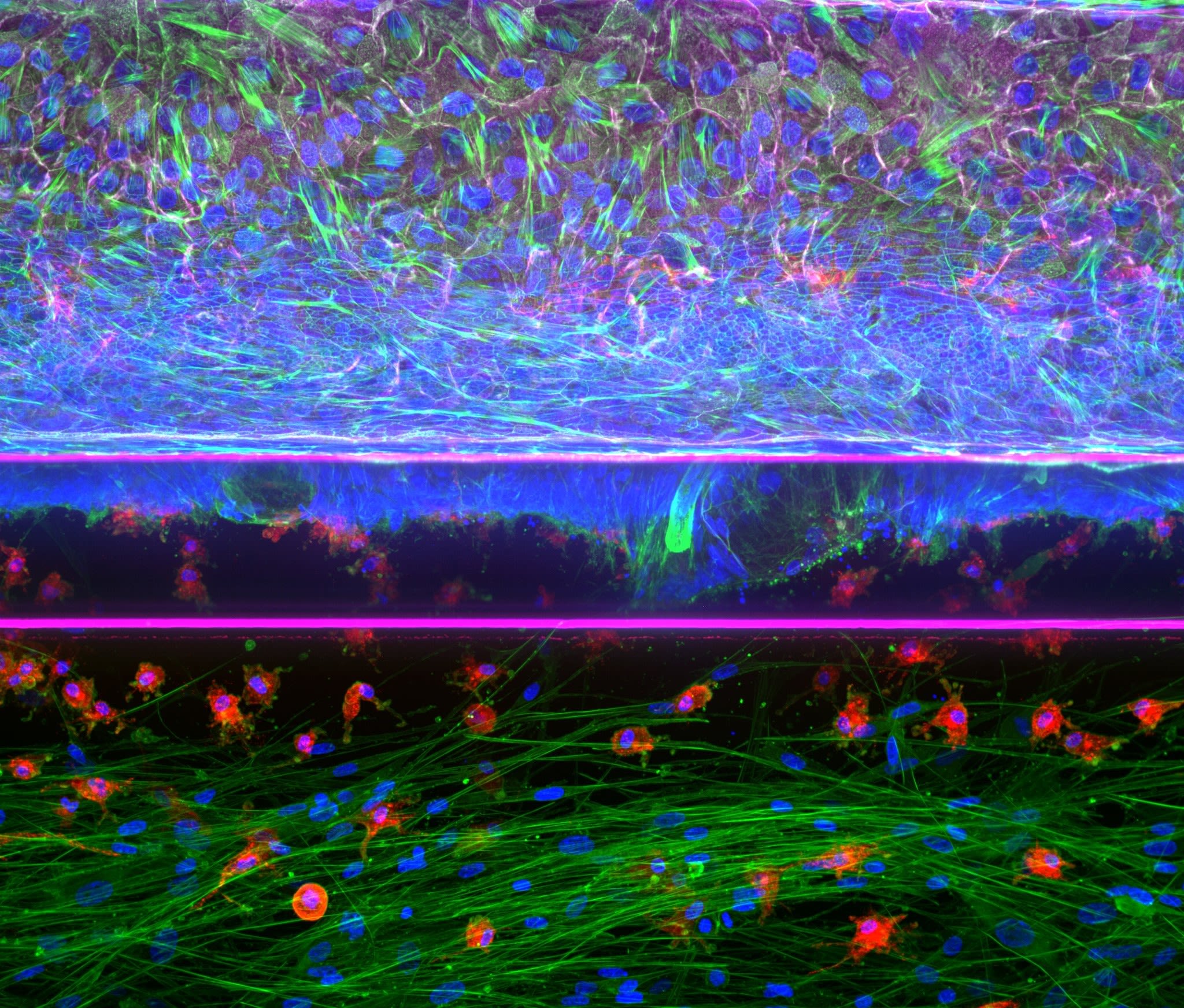
The translation of biomedical knowledge to patients and users results in new medicines, medical technologies, and food products. This benefits our health, our environment, and economy. Animal testing is currently an important link in the biomedical development process, but it is increasingly under pressure. Often, animal tests are not a good model for human application; they are time-consuming, expensive, and cause animal suffering. To continue improving the health of humans and animals, a radically different approach is needed: making the step to humans and animals faster and more effective, without relying on animal testing by default.
The new Ombion Centre for Animal-Free Biomedical Translation (Ombion), formerly known as the CPBT, will shape this approach, creating new business opportunities around animal-free technologies and biomedical translation. This allows the Netherlands to distinguish itself globally and realize its ambition of becoming a leader in animal-free innovation.
Aim
Ombion is a national centre for valorizing and disseminating animal-free innovations and expertise. It aims to improve and accelerate the transition of new biomedical innovations to patients and users, at lower costs, and without the use of animals. This will lead to safer, more effective, and better medicines while reducing animal testing.
Together with a large number of national and international partners, Ombion will work on the development and dissemination of animal-free biomedical innovations and expertise. The initial transition projects will focus on ALS, cystic fibrosis, osteoarthritis/rheumatic diseases, and asthma/COPD. Ombion will implement the available and developed methods, tools, and expertise together with researchers and companies. The new centre will also offer education, training, advice, and support to enhance the acceptance and use of animal-free biomedical innovations. Combined, Ombion will run an integrated program that accelerates the transition to animal-free testing and strengthens the Dutch economy.
Budget
The total budget available to Ombion in the period from 2025 to 2034 is 245 million euros. 124.5 million of this budget is an investment from the Dutch National Growth Fund (NGF); the other funds are contributed by public and private Ombion partners.
The translation of biomedical knowledge to patients and users results in new medicines, medical technologies, and food products. This benefits our health, our environment, and economy. Animal testing is currently an important link in the biomedical development process, but it is increasingly under pressure. Often, animal tests are not a good model for human application; they are time-consuming, expensive, and cause animal suffering. To continue improving the health of humans and animals, a radically different approach is needed: making the step to humans and animals faster and more effective, without relying on animal testing by default.
The new Ombion Centre for Animal-Free Biomedical Translation will shape this approach, creating new business opportunities around animal-free technologies and biomedical translation. This allows the Netherlands to distinguish itself globally and realize its ambition of becoming a leader in animal-free innovation.
Aim
Ombion is a national centre for valorizing and disseminating animal-free innovations and expertise. It aims to improve and accelerate the transition of new biomedical innovations to patients and users, at lower costs, and without the use of animals. This will lead to safer, more effective, and better medicines while reducing animal testing.
Together with a large number of national and international partners, Ombion will work on the development and dissemination of animal-free biomedical innovations and expertise. The initial transition projects will focus on ALS, cystic fibrosis, osteoarthritis/rheumatic diseases, and asthma/COPD. Ombion will implement the available and developed methods, tools, and expertise together with researchers and companies. The new centre will also offer education, training, advice, and support to enhance the acceptance and use of animal-free biomedical innovations. Combined, Ombion will run an integrated program that accelerates the transition to animal-free testing and strengthens the Dutch economy.
Budget
The total budget available to Ombion in the period from 2025 to 2034 is 245 million euros. 124.5 million of this budget is an investment from the Dutch National Growth Fund (NGF); the other funds are contributed by public and private Ombion partners.
News
Ombion celebrates launch and site opening
On 7 July 2025, the Centre for Animal-free Biomedical Translation, supported by the Dutch National Growth Fund, was officially launched under its new name: Ombion Centre for Animal-free Biomedical Translation. Read more
Press release on CPBT funding
The press release on the NGF investment in CPBT was published on the Utrecht University website and many CPBT partner websites. It was also shared by various external media channels. Read the press release here
ARD Tagesthemen television broadcast
German Public television channel ARD broadcasted an item showcasing examples of animal-free research and educational alternatives recorded at Utrecht University. View the broadcast on LinkedIn here (in German)
BNR Nieuwsradio podcast episode
BNR Nieuwsradio show 'Wetenschap Vandaag' created a podcast episode on the CPBT. Wouter Dhert (UU), Anne Kienhuis (RIVM) and Jeffrey Beekman (UMC Utrecht) were interviewed about CPBT and their research. Listen to the podcast here (in Dutch)
Ombion at Utrecht Science Park
Ombion will be established as a physical centre located at Utrecht Science Park, where expertise and infrastructure from leading Dutch knowledge institutions, research centres, and businesses come together to drive the transition to animal-free innovation. Ombion will serve as a one-stop shop for organisations interested in adopting animal-free biomedical translation or for those looking to conduct preclinical research in the most efficient way. Our approach is built on four interconnected components explained below, forming a comprehensive strategy to enhance biomedical research and translation. The centre will leverage the knowledge and tools developed through its transition projects, transforming them into marketable propositions and spin-outs, supported by in-house expertise.
How Ombion operates
Transition Projects: Within Ombion's transition projects, public and private partners will collaborate to develop new tools, methods, and knowledge to demonstrate the transition to animal-free biomedical translation in light of current biomedical challenges. The initial transition projects focus on ALS, Cystic Fibrosis, Osteoarthritis & Rheumatoid Arthritis, and Asthma & COPD.
Expertise Development: Alongside the transition projects, Ombion will implement an in-house advanced development program. This program will expand the knowledge acquired from the transition projects and cultivate new expertise, thereby strengthening each proposition and addressing existing challenges and hurdles. The Ombion's efforts will concentrate on four key areas:
- Transition science, ethics, and sustainability
- Regulatory innovation
- Educational program development
- Data science, supporting tools and models
Propositions: The methods, tools, and expertise developed within the transition projects are used to create propositions for academic researchers, companies, and governmental bodies. Ombion will provide the following propositions:
- Training and education on animal-free biomedical translation
- Advising on existing data, tools and methods for animal-free biomedical translation
- Providing support for the validation and adoption of animal-free tools and models
- Supporting the development of new animal-free methods, tools, and models.
Spin-out Activities: Ombion will support spin-outs emerging from the new products, methods, tools, expertise, and services developed within its transition projects. This support will include mobilising financing, drafting business plans, building strong teams, and integrating these spin-outs into the thriving animal-free innovation ecosystem.
Ombion at Utrecht Science Park
Ombion will be established as a physical centre located at Utrecht Science Park, where expertise and infrastructure from leading Dutch knowledge institutions, research centres, and businesses come together to drive the transition to animal-free innovation. Ombion will serve as a one-stop shop for organisations interested in adopting animal-free biomedical translation or for those looking to conduct preclinical research in the most efficient way. Our approach is built on four interconnected components explained below, forming a comprehensive strategy to enhance biomedical research and translation. The centre will leverage the knowledge and tools developed through its transition projects, transforming them into marketable propositions and spin-outs, supported by in-house expertise.
How Ombion operates
Transition Projects: Within Ombion's transition projects, public and private partners will collaborate to develop new tools, methods, and knowledge to demonstrate the transition to animal-free biomedical translation in light of current biomedical challenges. The initial transition projects focus on ALS, Cystic Fibrosis, Osteoarthritis & Rheumatoid Arthritis, and Asthma & COPD.
Expertise Development: Alongside the transition projects, Ombion will implement an in-house advanced development program. This program will expand the knowledge acquired from the transition projects and cultivate new expertise, thereby strengthening each proposition and addressing existing challenges and hurdles. Ombion's efforts will concentrate on four key areas:
- Transition science, ethics, and sustainability
- Regulatory innovation
- Educational program development
- Data science, supporting tools and models
Propositions: The methods, tools, and expertise developed within the transition projects are used to create propositions for academic researchers, companies, and governmental bodies. Ombion will provide the following propositions:
- Training and education on animal-free biomedical translation
- Advising on animal-free biomedical translation
- Providing support for the validation and adoption of animal-free tools and models
- Supporting the development of new animal-free methods, tools, and models.
Spin-out Activities: Ombion will support spin-outs emerging from the new products, methods, tools, expertise, and services developed within its transition projects. This support will include mobilising financing, drafting business plans, building strong teams, and integrating these spin-outs into the thriving animal-free innovation ecosystem.
Our partnership
At Ombion, a diverse consortium of academic scientists, lecturers and researchers from universities of applied sciences, industry partners, regulatory agencies, patient organizations, government bodies, and NGOs will collaborate to pave the way for more accurate and cost-effective medicines, minimizing the need for animal testing.
Core partners:
Utrecht University, HU University of Applied Sciences Utrecht, UMC Utrecht.
The Ombion Centre for Animal-free Biomedical Translation National Growth Fund application was submitted by the Ministry of Agriculture, Fisheries, Food Security and Nature (LVVN, formerly LNV) within the framework of its Transitie Proefdiervrije Innovatie (TPI) program.
Ombion partners (in alphabetical order):
3BrainAG, Allegro, ALS patiëntenvereniging, Amsterdam University Medical Center (AUMC), Aquilo, ArthroSave, Aryzon, AstraZeneca, Azar innovations, Chondropeptix, CHRN on-chip biotechnologies B.V, Citeq, Danone Nutricia Research, Eindhoven University of Technology (TU/e), Elevate Academy, Erasmus MC, ETB-bislife, EU JRC (EU Joint Research Centre, JRC), EU Reference Laboratory for alternatives to animal testing (JRC-ECVAM), FAIR Therapeutics, Genmab, GQ Bio Therapeutics, hDMT, hiPSC hotel LUMC, HIT CF Europe, HU University of Applied Sciences Utrecht, Humane Society International (HSI), InnoCore Pharmaceuticals, intoSURGERY, Johns Hopkins Center for Alternatives to Animal Testing (CAAT), Johns Hopkins University, Leiden University Medical Center (LUMC), Liposphere, Longfonds, Maastricht Universiteit (MU), Medicines Evaluation Board (CBG), Mimetas, National Institute for Public Health and the Environment (RIVM), NCFS, NeoVentures Biotechnology Europe, Netri, NLC, Nordic Bioscience, Optics11 Life, Orthros Medical, QurAlis, Radboud University (RU), Radboudumc, Recode Therapeutics, ReumaNederland, Roche, Sanofi, Stichting ALS Nederland, Stichting Proefdiervrij, Synapticure, Tecan, TNO, Treeway, UMC Utrecht, Unilever, University Medical Center Groningen (UMCG), University of Groningen (RUG), Utrecht University, VectorY, Wageningen University & Research (WUR), ZEISS & Arivis, ZonMw
Contact us:
For more information, please contact us at info@ombion-cpbt.nl
You can also follow us on LinkedIn and sign up for our mailing list
Postal address:
Ombion Centre for Animal-free Biomedical Translation
Yalelaan 62 (Life Sciences Incubator Building)
3584 CM Utrecht
The Netherlands










